The heat of vaporization for ethanol is 0.826 kJ/mol, a fundamental property that plays a crucial role in numerous industrial processes and everyday applications. Understanding this property is essential for optimizing energy efficiency, designing separation systems, and developing renewable energy technologies.
This comprehensive guide delves into the concept of heat of vaporization, its measurement techniques, and the factors influencing its value. We will explore practical applications and compare heat of vaporization values for different liquids, providing a thorough understanding of this important thermodynamic property.
Definition and Significance of Heat of Vaporization
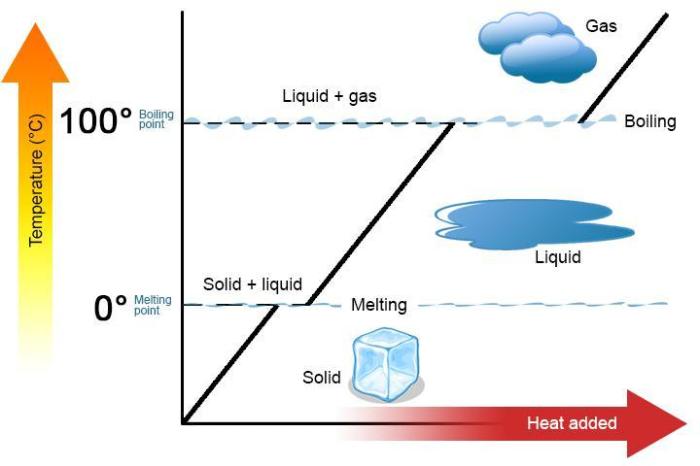
The heat of vaporization refers to the amount of energy required to convert a liquid into a gas at a constant temperature. It is a crucial property in understanding phase transitions and plays a significant role in industrial processes and everyday applications.
Measurement and Units of Heat of Vaporization
The heat of vaporization can be measured using various techniques, including calorimetry and differential scanning calorimetry (DSC). The units commonly used to express heat of vaporization are kilojoules per mole (kJ/mol) and joules per gram (J/g). The choice of unit depends on the context and the quantity of substance being considered.
Factors Affecting Heat of Vaporization
The heat of vaporization of a substance is influenced by several factors, including:
- Molecular structure: The molecular structure of a liquid affects the strength of intermolecular forces, which in turn influences the energy required to overcome these forces during vaporization.
- Intermolecular forces: The type and strength of intermolecular forces present in a liquid, such as hydrogen bonding, dipole-dipole interactions, and van der Waals forces, contribute to the heat of vaporization.
- Temperature: The heat of vaporization typically decreases with increasing temperature, as the molecules gain kinetic energy and require less additional energy to vaporize.
Applications of Heat of Vaporization
The heat of vaporization finds practical applications in various fields, such as:
- Refrigeration and air conditioning systems: Heat of vaporization is utilized in refrigerants to absorb heat from the environment, resulting in cooling.
- Distillation and separation processes: Heat of vaporization is used in distillation to separate liquids with different boiling points.
- Energy storage and renewable energy systems: Heat of vaporization can be used to store thermal energy in materials, such as molten salts, for later use in energy production.
Comparison of Heat of Vaporization Values, The heat of vaporization for ethanol is 0.826
The heat of vaporization values for different liquids vary significantly. The following table compares the heat of vaporization values for ethanol, water, and other common solvents:
| Liquid | Heat of Vaporization (kJ/mol) |
|---|---|
| Ethanol | 38.6 |
| Water | 40.7 |
| Methanol | 35.3 |
| Acetone | 29.1 |
The heat of vaporization values reflect the differences in molecular structure and intermolecular forces among these liquids.
Quick FAQs: The Heat Of Vaporization For Ethanol Is 0.826
What is the significance of heat of vaporization?
Heat of vaporization is crucial for understanding phase transitions and energy transfer processes. It determines the amount of energy required to convert a liquid into a vapor, which is essential in refrigeration, distillation, and other industrial applications.
How is heat of vaporization measured?
Heat of vaporization can be measured using calorimetry or differential scanning calorimetry (DSC). These techniques involve measuring the temperature change of a sample as it undergoes a phase transition, allowing for the calculation of heat of vaporization.
What factors influence the heat of vaporization?
The heat of vaporization is affected by molecular structure, intermolecular forces, and temperature. Stronger intermolecular forces, such as hydrogen bonding, result in higher heat of vaporization values.